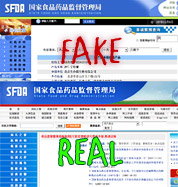
The Chinese government has discovered a fake diabetes medicine on a fake research institute website, which then links to a fake version of the official government health and drug watchdog agency’s site. If you’re paying attention to urls, it’s hard to not notice that something’s wrong—but we’re sure there’s more than enough people who don’t notice that little detail.
pharmaceuticals
Pfizer Launches Campaign To Warn Users Away From Generic Competitor
Pfizer is in panic mode about its rapid decline in Lipitor sales—in the last 18 months, it has dropped from 40% of the market for cholesterol-lowering drugs to 30%, and likely to drop further—so it’s launched a big media-blitz to convince people not to switch to simvastatin, the generic version of its name-brand competitor, Zocor. Zocor was more expensive than Lipitor, so Pfizer had nothing to worry about for years—but then Zocor lost its patent protection last year, and now doctors are switching patients from Lipitor over to Zocor’s generic twin to save money.

FDA Requiring Hearing Loss Warnings On Viagra, Cialis, Levitra
Stop doing that or you’ll go deaf! That’s the new warning (sort of) the FDA will require on popular anti-impotence drugs, spurred after a published report of a man who suffered sudden hearing loss after taking Viagra. The FDA took a look at side effect data and found 29 cases since 1996 where men suffered from similar hearing loss after taking one of the three drugs. “In two thirds of the cases, the hearing loss was ongoing, the agency said.” A drug to treat pulmonary hypertension, Revatio, will also receive the warning because it contains the same ingredient as Viagra.
Doctors Say Too Many Patients Can't Name The Drugs They Take
Too many patients don’t remember the names of the medications they’re on, posing problems for doctors who are trying to treat them, warn researchers at Northwestern University’s Feinberg School of Medicine in Chicago. About 40% of the patients surveyed “could not accurately recall what drugs they were taking,” and among those with “low health literacy,” the rate jumped to 60%.
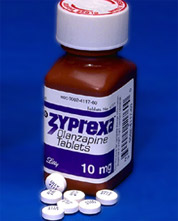
Lilly Caves, Agrees To Add Warnings To Schizophrenia Drug
Zyprexa, Lilly’s best-selling drug to treat schizophrenia, has been shown to cause “cause weight gain, high blood sugar, high cholesterol and other metabolic problems,” but until now, the company has refused to add any warnings about these side effects to the label. Now, sparked in part by lower sales, Lilly has announced that Zyprexa will warn consumers that it can cause high blood sugar. The American Diabetes Association claims that Zyprexa causes diabetes, but this isn’t addressed on the new warning labels.

FDA Might Create A "Behind-The-Counter" Drug Category
Next Month, the FDA will hold a public meeting to discuss whether or not they should allow certain drugs to be sold “behind-the-counter”—that is, after consultation with a pharmacist, but without the need for a prescription. If they move ahead with the plan, a new BTC category will be created, although what drugs will fall under it have not been determined.

Participating In A Clinical Study? You're On Your Own
The FDA’s own parent department, the Department of Health and Human Services, just issued a report that says the FDA “does very little to ensure the safety of the millions of people who participate in clinical trials,” according to the New York Times. The FDA has 200 inspectors, some of whom are part-time, to monitor 350,000 testing sites—and even when they reported “serious problems,” their findings were downgraded 68% of the time by higher-ups in Washington.

Liveblogging The House Energy And Commerce Committee Hearing On Food Safety
Starting today at 10 a.m., the powerful Chairman of the House Energy and Commerce Committee, John Dingell (D-MI), will hold a hearing on H.R. 3610, The Food and Drug Import Safety Act of 2007, or, as we have dubbed the bill, The Poison-Free Food Act. The bill would dramatically alter the FDA’s handling of imported foods, empowering the agency to:
- Issue mandatory recalls;
- Limit food imports to ports clustered near FDA inspection labs;
- Require a country of origin labels for food, drugs and medical devices;
- Subject exporters to a strict certification program administered by the Department of Health and Human Services.
The Committee will hear from two panels: The first will see FDA Commissioners and regulators defending their agency, while the second will host a panoply of foodies, including the Coalition for a Stronger FDA, the Center for Science in the Public Interest, the Grocery Manufacturers Association, and Big Pharma.

Antifungal Medication Makes You See Chewbacca
A new antifungal drug, voriconazole, causes patients to “develop a range of neurological side effects, including auditory and visual hallucinations,” within 24 hours to 2 weeks of beginning treatment. The drug is marketed as Vfend, and is administered intraveneously to treat serious fungal infections. The National Institute of Health has been testing the toxicity of the drug and reported the neurological side effects at a recent conference.

Is The "Wal-Mart Effect" Slowing Drug Inflation?
The inflation rate for prescription drugs—currently at 1 percent for the past 12 months—is at its lowest ever recorded in the past three decades, and some are speculating that Wal-Mart’s popular $4 generic drugs program is helping drive the costs down across the market.

House Approves Drug Testing Bill, Senate & Prez Expected To Follow
This week, the House of Representatives passed a new bill that gives the FDA the power to require new warning labels on existing prescription drugs, and the power to request “post-approval” studies of medicines as warranted. It also gives the FDA the authority to levy fines as high as $10 million to companies that fail to comply. The bill passed with a 405-7 vote on Wednesday and is expected to be passed by the Senate and approved by the President.
../../../..//2007/09/13/mental-floss-has-a/
Mental Floss has a fun quiz that asks you to match actual warnings to popular drugs. It’s a good way to brush up on your side effect trivia, so you’ll know what to take to increase your gambling addiction but not interfere with your sleep driving. (Sadly, we only got 3 out of 10 correct.) [Mental Floss via BoingBoing]

Senate Proposal To Allow Generic Versions Of Biotech Drugs After 12 Years
A Senate proposal would strip biotech drugs of their patent-protected status after twelve years, opening the door to competition from generic drug makers. Patent protection determines how long obnoxious pharmaceutical CEOs can spend outside their competitor’s offices dancing with their drugs to MC Hammer’s 1990 hit, “U Can’t Touch This.” Unlike regular drugs made by chemical synthesis, biotech drugs are derived from human proteins.
FDA Knew About Potentially Lethal Diabetes Drug Since Last August, Said Nothing
The study was outed yesterday on the New England Journal of Medicine’s website. The editors of the journal and the study’s lead author both warned that the research methodology left the “findings open to interpretation.”
Merck’s Vioxx Replacement Still A Heart Risk
Merck’s getting in on the arthritis market again with a new drug, called Arcoxia. You might remember their previous offering, Vioxx, which was discontinued two years ago after octogenarians countrywide lifted their contorted, claw-like hands to a withered chest and let out a rattling gasp under the influence of a massive, Vioxx-induced heart attack. Lawsuits abounded.

UPDATE: Parexel Destroys Immune Systems, Avoids Liability
You may recall the test subjects of drug trial TGN1412 who were left seriously maimed moments after taking the experimental drug.

MERCK Gets Mercked for $9 Million in Vioxx Suit
A jury awarded $9 million in punitive damages to John Darby who blamed his heart attacks on Vioxx. The sum is a defeat for drug-maker Merck, which, for some fucking reason, was assumed to be “bulletproof” because the trial took place in New Jersey.