FTC: No Evidence That Herbal Products Alleviate Opiate Withdrawal
Given the pain, nausea, intestinal distress, craving, and other unpleasantness involved in opiate withdrawal, it’s understandable that people might be tempted to put their faith in an herbal supplement that promises to alleviate these problems. However, the Federal Trade Commission says the marketers of one such product had no science to back up their claims.
The FTC filed a complaint [PDF] yesterday claiming that Catlin Enterprises Inc. and its CEO George Catlin violated the FTC Act by making unsubstantiated claims that a product called Withdrawal Ease significantly alleviated the symptoms of opiate withdrawal also significantly increased the likelihood of a person overcoming opiate dependency.
The FTC also accused the defendants of misrepresenting that clinical studies proved Withdrawal Ease’s effectiveness.
According to the agency, the company deceptively represented that another product, Recovery Ease, significantly alleviated long-term opiate withdrawal symptoms. Both products come in Daytime Formula and Nighttime Formula.
The daytime formulas are comprised of combinations of vitamins and minerals, and what product labeling identifies as “proprietary blends” of herbs and other compounds, the complaint notes. The Nighttime Formulas of both products are made up of different “proprietary blends” and do not contain vitamins and minerals.
On its site, the company claimed its Withdrawal Ease could target “specific brain functions in order to help ‘reboot’ your brain’s natural chemical balance and function.” When it comes to your actual internal organs, the product claimed to provide “comprehensive detox to rid the body of toxins and free-radicals as well as help treat many of the withdrawal symptoms that originate in the digestive system, liver, and musculature.”
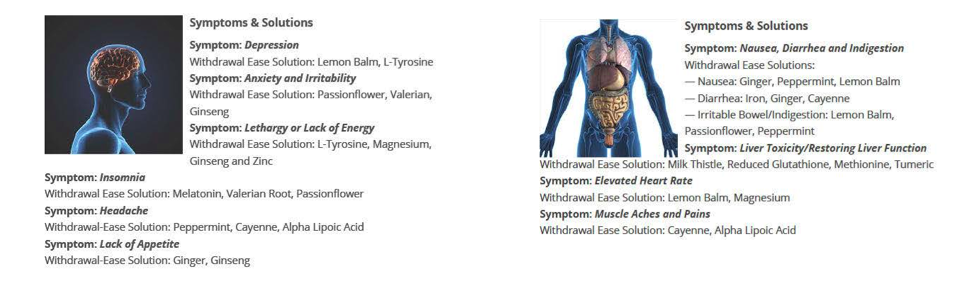
The site listed symptoms — both physical and mental — along with the ingredient included that purportedly eased those ailments.
For example, if you’re feeling depressed you’ll benefit from lemon balm and L-Tyrosine, whatever that is. Nausea? Ginger, peppermint, and lemon balm are supposed to take care of that.
A proposed court order [PDF] would settle the FTC charges, the agency announced today, and bars the company from making claims about opiate withdrawal, opiate dependency, or other health conditions, including through their product names, unless they possess competent and reliable science to back up those claims.
The order would also impose a $6.6 million judgment against the defendants, which will be suspended based on their inability to pay, the FTC says.
“Opioid addiction is a scourge that has affected millions of Americans,” said Acting FTC Chairman Maureen K. Ohlhausen. “People who struggle with this problem need real help, not phony claims and false promises like the ones peddled by these defendants.”
Want more consumer news? Visit our parent organization, Consumer Reports, for the latest on scams, recalls, and other consumer issues.

