Pfizer Recalls Antidepressant Effexor Because It Is Not A Heart Medicine
Pfizer says there is a very slim chance that some Effexor bottles may contain stray Tykosin capsules.
According to the recall notice, a pharmacist recently reported finding a capsule of Tikosyn inside one container of Effexor XR capsules. Tikosyn, also produced by Pfizer, is an antiarrhythmic agent given to cardiac patients with abnormal heart rhythms.
Accidental use of Tikosyn could result in serious adverse health consequences that could be fatal, says the FDA.
Pfizer says it knows of no other instances of Tikosyn being found mixed in with Effexor or Venlafaxine capsules, and believes the odds of a consumer unintentionally receiving a Tikosyn in with their other meds is unlikely. However, it is recalling these lots out of caution.
The FDA is asking pharmacists to immediately quarantine and return all recalled lots, as well as notify any customers to whom they distributed the recalled products. Patients with affected product should notify their physicians and/or return product to their pharmacies.
Patients with questions regarding this recall can contact Pfizer Medical Information at 1-800-438-1985 (Monday to Thursday 9am to 8pm ET or Friday, 9am to 5pm ET). Those who have questions about returning recalled capsules should contact Stericycle at 1-888-345-0481 (Monday to Friday 8am to 5pm ET).
Patients should contact their physician or healthcare provider if they have experienced any problems that may be related to taking this drug product.
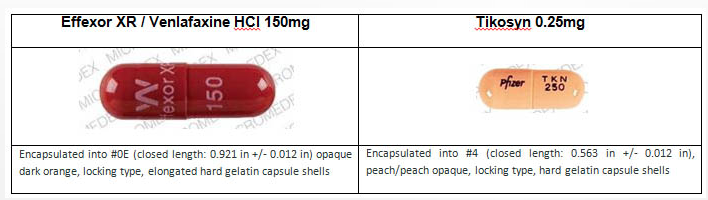
As you can see from the images above, Effexor and Tykosin are very different in appearance and size. The Effexor pills are nearly twice as long as Tykosin and come in dark orange capsules. Tykosin’s capsules are peach in color. If your Effexor bottle has an odd-looking capsule inside that doesn’t match the others, you should not take it and you should contact the pharmacy that filled the prescription.
Want more consumer news? Visit our parent organization, Consumer Reports, for the latest on scams, recalls, and other consumer issues.

