Charlie writes:
Yesterday I did the unthinkable. I went to Radio Shack.
Thanks for visiting Consumerist.com. As of October 2017, Consumerist is no longer producing new content, but feel free to browse through our archives. Here you can find 12 years worth of articles on everything from how to avoid dodgy scams to writing an effective complaint letter. Check out some of our greatest hits below, explore the categories listed on the left-hand side of the page, or head to CR.org for ratings, reviews, and consumer news.

Charlie writes:
Yesterday I did the unthinkable. I went to Radio Shack.

Unlike Nancy Nord (she’s the CPSC boss that tried to hint to Congress that her agency needed more funding through sly winks and interpretive dance numbers), the FDA chief is ignoring Bush’s “do not ask for more money” rule and demanding more funds.

Hooray! Steve Warshak, the snake oil salesman responsible for Enzyte (and consequently for those awful “Smiling Bob” ads) was found guilty today of conspiracy to commit mail fraud, bank fraud, and money laundering. So was his mom.
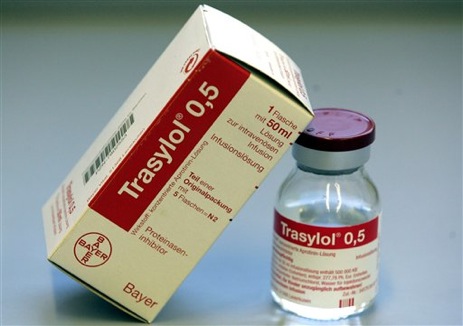
The FDA yanked the heart surgery drug Trasylol off the market last November, but a medical researcher now claims that 22,000 lives could have been saved if Bayer AG hadn’t withheld the results of an earlier internal study proving the drug’s danger. An FDA committee held hearings in September 2006 to determine Trasylol’s safety, but three of the committee members had a financial interest in Bayer, and the drug maker had underwritten the committee chairman’s research.

NY Magazine has published a interesting personal account from a patient who was taking the smoking-cessation drug Chantix. The FDA has reported 37 suicides and more than 400 reports of suicidal behavior in connection with Chantix, a pleasure blocking drug that sits in the nicotine receptors and prevents the smoker from properly experiencing their nicotine high. The FDA recently issued a patient advisory about the drug, requesting that patients carefully monitor their moods. The possible side-effects of Chantix now include “anxiety, nervousness, tension, depressed mood, unusual behaviors and thinking about or attempting suicide.”

Here’s a little free advice from your friends at The Consumerist: Don’t deposit bags of meth at the ATM. You don’t get any interest and they’re probably going to figure out who are after they see your name and account number.

A coffee shop in Montreal has removed a “dud” security camera from its bathroom after news of it hit the local papers. Corporate headquarters asked the franchise owner to take it down, and apologized/avoided blame in a press release that said they were “not consulted in advance.” The franchise owner had installed it as a sort of junkie scarecrow, to frighten away heroin users who were leaving dirty needles in the bathroom stall.

Last night’s commercials were a tame batch of disappointment. Everybody wanted cutesy animals—squirrels, horses, ponies, pigeons, crickets, dogs, lions, and lizards—to endorse their products. After the jump, the four spots that caught our eye.

The U.S. Institute of Medicine called on Congress today to “establish a single national resource of health information.” The resource would collect all available data on every drug in the marketplace, and be available to consumers to educate themselves about any and all possible treatments in order to make better-informed decisions with their doctors.
../../../..//2008/01/17/a-clinical-trial-of/
A clinical trial of Zetia, a popularly prescribed cholesterol-lowering drug, “failed to show that the drug has any medical benefits.” In fact, fatty plaques grew almost twice as fast in patients who took Zetia along with Zocor in a combo product called Vytorin. However, “patients who are taking Vytorin or Zetia should talk to their doctors if they are concerned and not discontinue taking the medicines on their own.” [New York Times]
A new study—”the most thorough to date,” writes the New York Times—shows that about a third of the studies for some of the market’s most successful antidepressants (Paxil, Prozac, Zoloft, Effexor) were never published because they didn’t have favorable results. “While 94 percent of the positive studies found their way into print, just 14 percent of those with disappointing or uncertain results did.” The implication is that the makers of these drugs intentionally misled consumers and the federal government on their effectiveness.

This personal testimony about health supplements from winstonthorne on today’s earlier post is too good—and disturbing—to leave buried in comments:One of my friends actually stuffs capsules for a living for a company making an herbal “sexual stimulant” – she literally sits there on her…

A new article published today in Clinical Cancer Research says that two men “developed aggressive and incurable prostate cancer within months of taking the same supplement.” The doctors examined the supplement and discovered it contained testosterone and estradiol, and “when they tested it on tumor cells in the lab, they found it fueled the growth of prostate cancer cells more potently than testosterone alone.” Either don’t take herbal/hormonal dietary supplements, they urge, or make sure you fully disclose to your doctor what you’re taking.

Jury selection began today for the federal trial against the man, his mom, and the business associates responsible for the “male enhancement” supplement Enzyte, reports WKRC in Cincinnaaa-ti. The charges against Steve Warshak and his Berkeley Premium Nutraceuticals company include “committing wire and mail fraud, money laundering, and misbranding.” No mention of creating what’s possibly the world’s most irritating TV ad, but we guess that crime is so great that it’s being left for hell to sort out.

Two former sales reps for the pharmaceutical company Amgen are suing “for lost wages and other compensation after refusing to participate in improper promotion of the company’s blockbuster psoriasis drug Enbrel.” They claim that Amgen encouraged them to “illegally access patient records to induce insurance carriers to pay for the pricey drug,” according to their attorney. Amgen promptly responded that the suits were without merit, and then handed out blister packets of popular drugs, branded desk calendars, and free t-shirts, so everything’s cool.
It’s okay for drug companies to spend oodles on advertising because they spend even more making sure their drugs are safe and effective, right? Not so much, according to a study in PLOS Medicine.

If you’re black, Hispanic, or “Asian/other,” you might want to make sure your voice is heard loud and clear the next time you have to make a trip to the ER. Research published in the Journal of the American Medical Association shows that over the past 13 years, white patients were prescribed powerful opioid painkillers 31% of the time, versus 23% for blacks, 24% for Hisanics, and 28% for Asians and “others.”

Today the FDA announced that a group of Chinese “health supplements” from Puerto Rican-based Shangai Distributor, Inc., contain undeclared sildenafil, the active drug in Viagra, and are therefore illegal. The supplements are named Super Shangai, Shangai Ultra, Shangai Ultra X, Lady Shangai, and (perhaps the best name of the product line) Strong Testis. Shangai Regular, also known as Shangai Chaojimengnan, was found to have “an unapproved substance with a structure similar to sildenafil that may cause similar side effects and drug interactions,” and is therefore also included on the warning list.
![]()
Part of ![]()
Founded in 2005, Consumerist® is an independent source of consumer news and information published by Consumer Reports.
