Some EpiPen Injectors Recalled, But Not In The U.S. Image courtesy of M
Four batches of EpiPen auto-injectors have been recalled by the manufacturer over concerns that the devices may fail to work when needed. However, Mylan — the company behind the emergency allergy treatment — tells Consumerist that the potentially defective injectors were not distributed in the U.S.
Mylan’s Australian subsidiary AlphaPharm Pty. announced the recall yesterday, saying a manufacturing issue “may result in patients not receiving the required dose of adrenaline,” which can lead to the user’s symptoms getting worse, with potentially lethal results.
The company published this list of batch numbers and expiration dates for the four recalled batches:
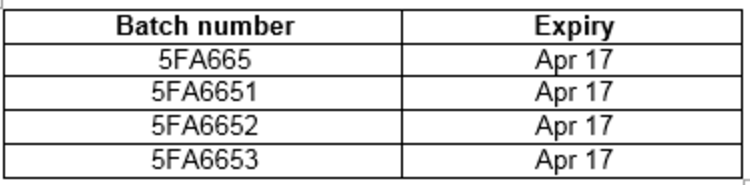
This information can be found on the end of the carton containing EpiPen packs and printed on the label of each pen.
Mylan says it knows of two instances “world-wide” where the EpiPens from the recalled lot failed to activate. Since this implies that the recalled injectors were distributed internationally, we asked the company why it was not announcing the recall in the U.S.
“Mylan has issued a voluntary recall for one lot of EpiPen Auto-Injectors distributed in Australia, New Zealand, Europe, and Japan only,” said the company in an emailed response to Consumerist. “This lot was not distributed in the U.S.”
That said, it certainly can’t hurt to check your stash of EpiPens to make sure they aren’t somehow included in the recalled batches. We also have a number of readers outside of the U.S., so we’re posting this news as a service to any of them who may have some EpiPens waiting to (hopefully never) be used.
Pfizer’s Meridian Medicial subsidiary manufactured the EpiPens in the recalled lot. The company referred questions about the recall to Mylan.
Want more consumer news? Visit our parent organization, Consumer Reports, for the latest on scams, recalls, and other consumer issues.

