Ever since the FDA and Congress started asking Johnson & Johnson to explain why it keeps recalling medicine, there have been references to an unpublicized “recall” that happened in November 2008. Last month, at a hearing of the House Oversight and Government Reform Committee, a J&J executive swore that the company didn’t mean to mislead anyone. It turns out that wasn’t exactly accurate: Bloomberg has obtained emails from J&J’s company, McNeil Consumer Healthcare, that show executives knew the secret recall would trigger an FDA reaction if the agency got wind of its full scope. [More]
zyrtec
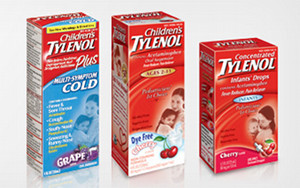
FDA Announces Widespread Investigation Of McNeil After Tylenol Recalls
Remember the recalled liquid Tylenol and other children’s medicines last month? Or the stinky drugs that were recalled back in January? Or the children’s Tylenol that was recalled last September? The FDA remembers, which is probably why it’s “conducting a company-wide investigation of McNeil Consumer Healthcare’s drug manufacturing practices to determine whether similar problems exist throughout the company.” Also, a date has now been set (May 27) for the House Committee hearing where the CEO and chairman of parent company Johnson & Johnson are expected to testify. [More]
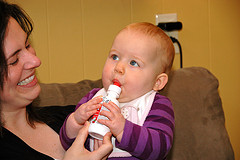
Children's Tylenol, Motrin, Other Medications Recalled
Head to your medicine cupboards and start checking out the children’s meds you might have there — the Food and Drug Administration announced today Johnson & Johnson’s McNeil Consumer Healthcare Unit has begun a voluntary recall of various medicines because of manufacturing discrepancies. [More]

Giant Foods Cashier Said I Had To Be 30 To Buy Zyrtec!
Reader Amie had an odd encounter with a Giant Food cashier today. As she was checking out the cashier asked if she was 30 because he wasn’t supposed to sell Zyrtec to anyone under 30.

Zyrtec Goes Behind-The-Counter
Zyrtec users can now buy it without a prescription—but they’ll have to show ID because it going to be sold from behind the counter with the other meth supplies. [Associated Press]


