Since introducing ResearchKit, its open-source framework for scientists to develop iPhone apps for medical research, Apple has made a few tweaks to the submission guidelines for apps that aim to collect and use sensitive medical data. One new addition is that anyone submitting an app that does research on humans must submit proof that the study has been approved by an independent ethics review board. [More]
researchkit

Apple Clarifies Requirements For Medical Research Apps
Earlier this week, Apple announced HeathKit, an open-source software framework to help medical researchers use iPhones to gather data for medical research. This raised some concerns about researchers’ plans to share data collected from the apps, as well as consent and privacy. Now Apple has revised their App Store guidelines before the kit launches, but is that enough to keep study participants informed and safe? [More]
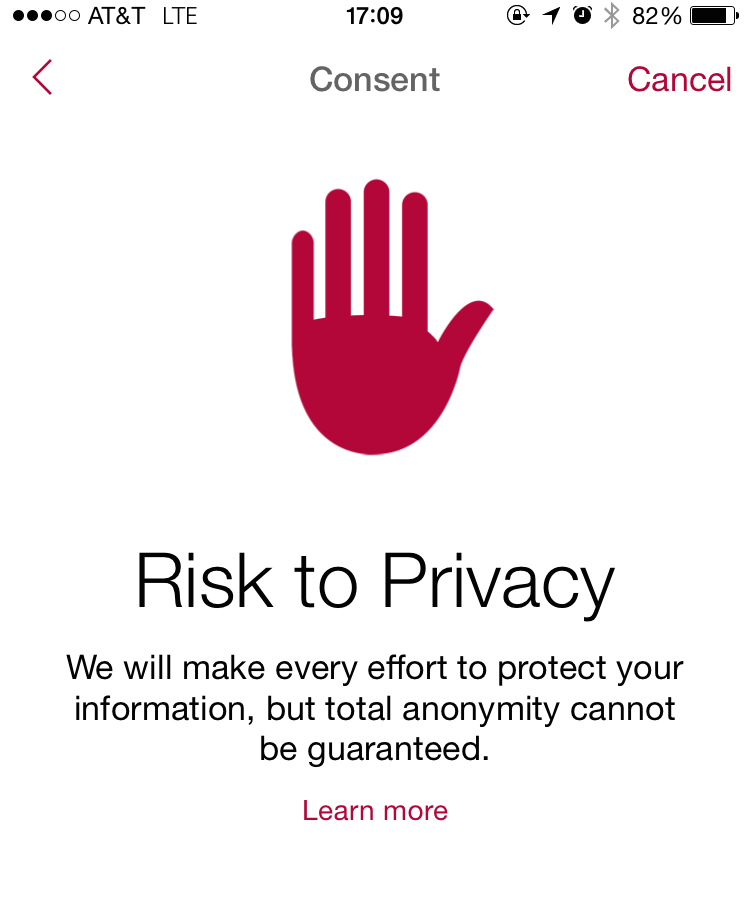
The Privacy And Consent Issues With Apple’s New ResearchKit
Earlier this week, Apple gave us wrist computers and took away almost all of the ports in its notebook computers, and also announced something that gadget fans may not have expected: a set of apps called ResearchKit designed to help medical researchers collect data from ordinary citizens for their research. Tens of thousands of people have already signed up for studies, which is potentially great for science. Is it good for us, the potential research subjects, though? [More]


