Do Food Regulators Care If Foreign Farmers Use Veterinary Drugs Banned In The U.S.?
In a letter [PDF] to the FDA, USDA, and the Office of the United States Trade Representative, the advocates — Consumers Union, Center for Science in the Public Interest, and Food Animal Concerns Trust — asks that the U.S. delegates to the upcoming meeting of Codex Alimentarius Committee on Residues of Veterinary Drugs in Food use their leverage to urge other countries to stop using nine drugs that have already been prohibited for use in this country.
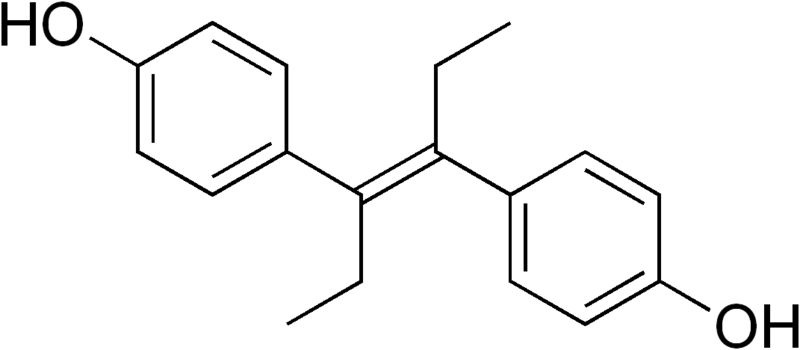
For the drugs in question — carbadox, nitrofural, furazolidone, chlorpromazine (thorazine), stilbenes (e.g. diethylstilbestrol, DES), olaquindox, dimetridazole, ipronidazole, metronidazole, and ronidazole — the Codex’s Joint Expert Committee on Food Additives (JECFA) has not been able to identify an acceptable residue level that may not put human beings at risk. The group is meeting to decide whether to set the global standard that these drugs should simply not be used, or opt for a lesser standard that would still allow their use.
Of the drugs on the list, only carbadox — which has been shown to be carcinogenic — is still permitted for use in the U.S.
“These drugs have been prohibited for use on animals in the United States for many years,” said Michael Hansen, Senior Scientist at Consumers Union. “Among them are [estrogen analogue] DES, which is well known for causing cancer in the daughters of women who took the drug when pregnant, and the antipsychotic drug thorazine.”
DES was used for decades as a feed additive to encourage growth in farm animals, but has been banned from use in poultry farming since 1959 and prohibited from use in feed for cattle and sheep since 1979.
“These drugs are not needed for animal health and most countries have adopted safer alternatives,” said Steven Roach, Public Health Program Director of the Food Animal Concerns Trust.
But the advocates claim the U.S. delegates to the Codex conference have yet to take a hard line on the use of these drugs, instead urging that countries should be allowed to continue using these veterinary drugs as long as they do not leave “residues of toxicological concern,” the lesser of the two options before the JECFA.
“We believe the U.S. position on these Codex standards potentially put U.S. consumers at risk, when they eat imported meat and fish or eat these foods when they travel, as well as consumers in other countries,” Hansen said.
One roadblock to getting U.S. support is that tenth drug on the Codex agenda, carbadox, which is still allowed to be used in pig-farming in the U.S. (even though its use has been completely banned in European Union countries).
If the U.S. delegates support a global ban on the nine drugs, they would possibly have to accept a ban on this one. So don’t be surprised if the American representatives at the conference in Minneapolis chicken out and support the flimsier of the two options.
Want more consumer news? Visit our parent organization, Consumer Reports, for the latest on scams, recalls, and other consumer issues.