Weight-Loss Drug Alli Recalled Over Tampering Concerns
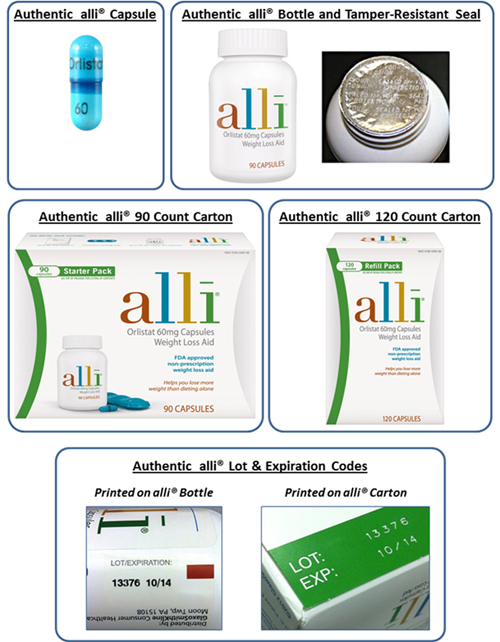
GSK has released these photos of what authentic alli products should look like so that customers can be on the lookout for tampered-with packaging and fake products.
According to GSK, it received inquiries from consumers in seven states about bottles of alli that contained tablets and capsules that were not actually alli, but were “A range of tablets and capsules of various shapes and colors.” The company also has reports about alli with missing labels and fake tamper-evident seals. These bottles were sold at retail stores and contained in boxes that appeared to be authentic alli packaging.
Authentic alli capsules are turquoise blue with a dark blue band imprinted with the text “60 Orlistat.” The inner foil seal on an alli bottle is imprinted with the words: “Sealed for Your Protection.”
Consumers should confirm any alli® in their possession matches this description. Pictures of the product are available on our website: http://www.myalli.com.
GSK says it is investigating the matter and is working with the FDA on the recall. In the meantime, alli users should check their capsule inventory for evidence of tampering. Consumers who believe they have questionable product or have any concerns should contact GSK promptly at 800-671-2554. If you have taken a pill, tablet, or capsule that you believe wasn’t authentic alli, you should contact your healthcare professional.
The company has this website with more information about the product and the recall.
“Safety is our first priority and we are asking retailers and pharmacies to remove all alli from their shelves immediately,” said Colin Mackenzie, President Consumer Healthcare North America. “We are committed to finding out what happened and to doing everything possible to prevent future issues with alli®,” said Mackenzie. “We regret any inconvenience caused by this retailer recall.”
Want more consumer news? Visit our parent organization, Consumer Reports, for the latest on scams, recalls, and other consumer issues.

