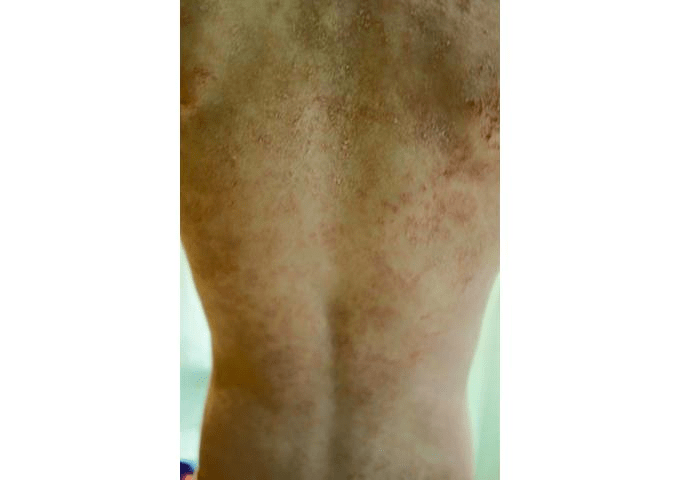
On Lucy’s* eighth birthday, she woke up to a surprise. It wasn’t a cool toy or a fun game, the kind of things kids often look forward to on days like this. Instead, her back and stomach itched so badly she was crying.
The small rash on her back spread quickly, while Lucy endured more misery and discomfort. According to her father, the young girl said, “Daddy, if this is living, I don’t want to live anymore.”
It was clear to her family that Lucy was suffering. But why? What was the source of this sudden attack?
The answer: a common preservative found in personal care products like body wash and shampoo; there’s a good chance you could walk into your bathroom right now and find any number of bottles that contain it. It’s called methylisothiazolinone [MI], and it made Lucy’s life hell for more than four months.
A Long, Uncomfortable Road To The Truth
Reactions like Lucy’s to MI are statistically rare, but examples are not difficult to find. There is, for example, a Facebook group for those who are sensitive to MI (as well as related preservatives isothiazolinone and methylchloroisothiazolinone, but more on those later) that has upwards of 4,500 Facebook followers. Symptoms can include redness, dryness, a burning or stinging sensation, facial swelling, blisters and crusting.
“…She basically didn’t sleep… It was like having a newborn, but a newborn who could talk and tell you just how miserable they were.”
Like most people, Lucy’s parents hadn’t heard of MI at the end of April 2014, when Lucy’s troubling rash first appeared. It took four months for the family to determine the culprit, an entire summer of watching Lucy struggle not to scratch between appointments with doctor after doctor.
“It was awful,” Lucy’s father John* told Consumerist. “Maybe the first month, she basically didn’t sleep. She’d fall asleep for 45 minutes… it was like having a newborn, but a newborn who could talk and tell you just how miserable they were, and there’s nothing you could do.”
Treatments ran the gamut of hospital-administered steroids to oatmeal baths. “You name it,” John said, but nothing seemed to be working and physicians could not explain the rash’s cause.
The search began with Lucy’s pediatrician who, according to John, said Lucy’s was the “worst rash she’s ever seen.”
Adding to the difficulty was the fact that the rash covered Lucy’s entire body from the neck down.
“You couldn’t see her skin,” he explains. “When they were trying to do blood tests, they couldn’t find the vein because they couldn’t see through the skin, it was covered with red bumps.”
After Lucy’s pediatrician, John says they tried a pediatric dermatologist and a slew of other specialists: an allergy immunologist, infectious disease experts, geneticists and rheumatologists. Lucy’s chest was X-rayed to rule out Hodgkin’s, another scare her parents were forced to endure.
“We had no idea what was causing this. She’s suffering, we’re wondering… does she have some kind of chronic condition?” John recalls.
As Lucy tells it, living with the rash was a constant battle for the eight-year-old.
“It’s like my body was telling me to scratch and I couldn’t resist it,” Lucy told Consumerist, after her parents said we could speak with her. “I couldn’t think against it. It was like a voice inside my head saying, ‘Scratch it! SCRATCH IT!’ I couldn’t resist it because I couldn’t think back at it and tell it not to.”
In an attempt to rule out a gluten-intolerance issue, Lucy’s parents had her avoid foods with gluten for months. Not a winning idea, especially for an 8-year-old who loves pizza.
“Because they thought that gluten was causing the rash, I had to stop eating gluten!” Lucy told Consumerist. “Which was like the one thing that made me feel better a little.”
The only positive, she says, was that she somehow didn’t get any of the rash on her face.
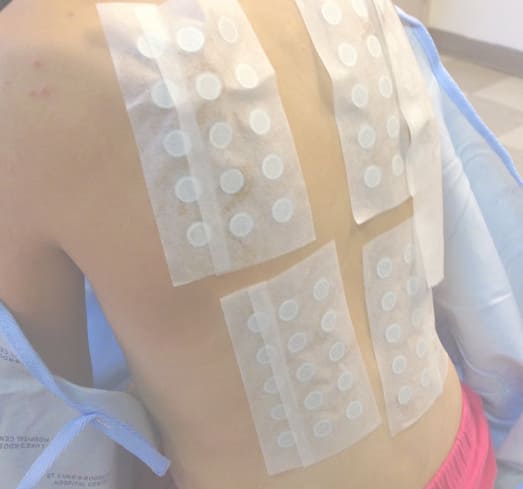
Finally, around the beginning of June, Lucy’s doctors prescribed powerful oral steroids and her discomfort lessened, even though the rash lingered. For months it still remained too severe for doctors to perform a patch test. A patch test involves applying different allergens to the skin in a controlled way and then monitoring the tested areas for reactions. The catch is that you need enough healthy skin to test, and Lucy’s entire body was covered with the rash.
When enough of Lucy’s skin was clear, her family went to see Dr. Vincent Deleo, then the head of dermatology at St. Luke’s Roosevelt in Manhattan. According to John, Deleo tested Lucy for sensitivity to around 70 different allergens and, at last, finally reached a conclusion: Lucy had a severe response to methylisothiazolinone.
At home, her parents tracked down the source of the MI — Suave Kids Body Wash, a product made by Unilever and marketed as “hypoallergenic” and “safe for kids’ delicate skin.”
“Our hypoallergenic, ophthalmologist-tested Suave Kids® Free and Gentle Body Wash is dye-free and will not irritate your child’s skin,” the product description reads, in part. “Made specifically for kids’ skin, it helps make bath time tear-free.”
Lucy would probably disagree with that part about the tears.
Upon realizing that methylisothiazolinone was causing Lucy’s awfully itchy symptoms, the family stopped using the Suave body wash — and the rash quickly cleared up. Finding the source was a huge moment, says John, even though it was hard to believe that the culprit had been sitting in their bathroom for all of these months, and that Lucy had been using it during her months-long ordeal.
“It was such an extraordinary relief,” he recalls. “Through the process, taking her for a chest X-ray for Hodgkin’s… you kind of think like, ‘This has got to be a low chance, but some kids get cancer.’ And she has this really weird condition and we’ve ruled out so many other things, a very prominent doctor is recommending we check it out…”
Thinking about it now, John tells Consumerist that it feels “so stupid” that they hadn’t thought to focus on that product, mostly because of the “hypoallergenic label” and words like “free and gentle.” They’d continued to use the product during the entire process, because of those words.
Why Is Methylisothiazolinone In So Many Products?

There’s a good chance you’ve never heard of MI or noticed it on product labels, but the chemical is increasingly being used as a preservative.
Cosmetic and skin care products have long used a class of preservatives known as parabens, but with some consumers calling on manufacturers to discontinue the use of parabens because of alleged long-term health concerns, Dr. Deleo tells Consumerist that these companies had to come up with something else to use to make things like body wash and shampoo shelf-stable.
About 25 years ago or so, Deleo tells Consumerist, many companies started using a new preservative to keep bubbly products bubbly, combining similar chemical methylchloroisothiazolinone (MCI) with methylisothiazolinone in a concentration of three-to-one.
“It was known that that could be an allergen,” he explains, “but that it was fairly safe to use in things like shampoos, which are washed off and so diluted,” in comparison to leave-on products like lotion.
“You can’t say it’s really common, I guess, but it’s certainly not rare.”
And then, for reasons unclear to Deleo, companies started using methylisothiazolinone alone and at a higher concentration to make it effective.
People started having reactions to it — but dermatologists often missed it, because previous patch-testing methods had only tested for the combination chemical, Deleo explains.
“So to make matters worse, not only was it occurring seemingly much more frequently than the combination, but we were missing it because we had the wrong allergen to test to,” he says.
Now, he says, he and other dermatologists test for both, and in his experience, it shows up pretty often.
“You can’t say it’s really common, I guess, but it’s certainly not rare,” Deleo tells Consumerist.
Seek, And Ye Shall Find

Despite some complaints linking MI to rashes, the chemical is so widely used that you’re likely applying it to your skin and hair on a regular basis.
After first talking to John about Lucy’s ordeal, I immediately looked in my bathroom to check my Suave shampoo and conditioner. Lo and behold, there was MI.
Though it’s more common to see MI in wash-off products, it’s also used in some leave-on products, including some Eucerin lotions and moisturizers made by Beiersdorf.
Between 2007 and 2010, the number of products that used it more than doubled to about 2,400 items, according to an estimate from the American Contact Dermatitis Society.
A quick check of a Rite Aid store in Brooklyn turned up three kids-themed body washes made by a company called MZB Accessories — a Frozen bottle, Spongebob Squarepants brand and a Spider-Man version — with both methylisothiazolinone and its sibling, methylchloroisothiazolinone on each label.
A Baylor College of Medicine study published in August 2014 evaluating products specifically marketed for babies and children found that out of 152 products surveyed at major retailers, 30 products — from facial or body wipes to hair products, bubble baths, moisturizers and sunscreens made by major brands — marketed specifically to the younger set contained MI.
“Of note, products marketed as ‘gentle,’ ‘sensitive,’ ‘organic,’ or ‘hypoallergenic’ often contained MI, thus emphasizing the importance of consumer scrutiny of product choices,” the researchers noted. “These findings reinforce the importance of educating parents and providing consumer decision-making advice regarding common skin care products, in order to help prevent [allergic contact dermatitis] in children.”
Speaking Of Hypoallergenic…
As for labels that use words like “natural” and “hypoallergenic,” Deleo says those terms mean nothing and shouldn’t guide your buying choices.
“Things like ‘natural’ and ‘hypoallergenic’… mean absolutely nothing.”
Indeed — even the federal government notes that “there are no Federal standards or definitions that govern the use of the term ‘hypoallergenic.'” The term means whatever a particular company wants it to mean… The term ‘hypoallergenic’ may have considerable market value in promoting cosmetic products to consumers on a retail basis, but dermatologists say it has very little meaning.
“Pay no attention whatsoever to things like ‘natural’ and ‘hypoallergenic’ because they mean absolutely nothing,” Deleo advises, pointing out that, “Pneumococcal pneumonia is also natural. So is poison ivy.”
The safest thing to do? Deleo recommends simply buying products that have very few ingredients.
If you’re worried that you might get a rash from methylisothiazolinone — people with eczema, for example, could be more susceptible — Deleo says it’s best to just stay away from products that contain that ingredient, or the lower concentration version of methylchloroisothiazolinone (it’s common to see them both together).
Even if you’ve never experienced a reaction, your skin can become sensitized over time, he says, causing problems later.
What’s Next?
Methylisothiazolinone has caught some flack in the last few years, most noticeably earning the title of Contact Allergen of the Year in 2013 from the American Contact Dermatitis Society. In 2013, Kimberly-Clark also came under fire for using MI in its Huggies baby wipes, and subsequently stopped using the preservative in those wipes.
(As Deleo explains, using MI in any areas that usually stay covered — like the perianal and genital regions — can increase sensitivity to the chemical, as absorption is increased.)
Deleo notes that many countries in Europe have already started restricting the use of MI, and he thinks U.S. companies have also started moving away from using it as an ingredient.
In 2013, the same year the ACDS named methylisothiazolinone as Contact Dermatitis Allergen of the year, the Scientific Committee on Consumer Safety, a European advisory group, said that MI should be used only in limited quantities for wash-off products, and that “no safe concentrations” existed for leave-on products like lotions, the New York Times reported in January of this year.
Other companies like Johnson & Johnson and Unilever have recently pledged to phase out using MI in new leave-on products.
A Johnson & Johnson spokesman told the NYT that the company would not use MI in new leave-on products, and is “evaluating its use in any new products that rinse off,” adding that it only uses MI when “absolutely necessary.”
In January 2014, an industry group called the Personal Care Products Council issued a statement acknowledging reports about “increased sensitization resulting from greater use of MI alone as a preservative.”
While noting that the Cosmetic Ingredient Review (CIR) Expert Panel, “an independent body of scientific and medical experts that assesses the safety of ingredients used in cosmetics in the U.S.” (that the PCPC itself established in 1976 with the support of the Food and Drug Administration and the Consumer Federation of America) limits MI as a preservative in cosmetics and personal care products up to 0.01% (100 ppm), the group said in light of new data, its members were “evaluating information relevant to exposure to MI as used in North America” with the goal of reporting those findings to the CIR.
After reviewing “relevant animal and human data,” the subsequent CIR report [PDF] said its panel “concluded that MI is safe for use in rinse-off cosmetic products at concentrations up to 100 ppm and safe in leave-on cosmetics products when they are formulated to be non-sensitizing, which may be determined based on a QRA [quality risk assessment].”
Consumerist also reached out to MZB Accessories (the distributors of those kid-themed body wash products I found on the Rite-Aid shelf), Breiersdorf (maker of Eucerin and Nivea products) and Galderma Laboratories (Cetaphil) for their policies concerning MI. We’ve yet to receive responses from these companies.
But even if manufacturers phase out MI, personal care products could still be hanging out in your bathroom or in a cabinet for years to come, depending on how often you go through body wash, shampoo and conditioner. If you buy these products in bulk, it could be quite some time before you exhaust your supply.
It also won’t necessarily be easy for companies to come up with alternative preservatives, despite the fact that they’re developing new preservatives all the time.
“When you test a product… even if you test it in a thousand people…there are going to be a lot of people who are going to get exposed and get allergic,” Deleo notes. “So it’s hard to pre-market test to get something that’s totally, totally safe.”

The front and back labels for the Suave product Lucy had been using when her rash appeared. Click image to enlarge.
“Methylisothiazolinone (MI) is a common preservative used in the personal care industry, where there are now allergy concerns. We have already removed MI from the vast majority of our leave-on personal care products and will complete reformulation of remaining leave-on products by the end of 2015.”
“For rinse-off products, we are reducing MI levels to ensure we meet any new EU regulatory requirements and we will implement this globally,” the statement continues. “The presence of MI is shown in the ingredients list so that any person who is allergic to MI can avoid using the product. We will continue to monitor scientific developments to ensure that all our products are safe, effective and fully compliant with regulatory requirements.”
John and his wife have not yet been in touch with Unilever regarding the specific “hypoallergenic” label on products with MI. He tells Consumerist he felt calling a consumer hotline number wouldn’t accomplish his goal of getting the company to stop using MI, and/or convince it to stop labeling products as “hypoallergenic.”
Instead, he filed a complaint with the Food and Drug Administration’s Center for Food Safety and Applied Nutrition (CFSAN).
“I didn’t get the sense that the FDA took my report seriously or that they’re going to take swift, meaningful action on this issue,” he told Consumerist.
Again, though Unilever and John have not been in touch, on the general topic of the “hypoallergenic” label on products containing MI, Unilever said:
“For Unilever products that make the claim ‘hypoallergenic’ we follow all industry standards for testing. The use of the term ‘hypoallergenic does not prevent the possibility that some consumers may have an allergic reaction, however the average population tested with sensitive skin are not shown to have allergic reactions. We will continue to engage with dermatologists on our approach, ensuring that our products are safe and effective.”
An Unforgettable Ordeal
Talking to Lucy these days, it’s clear she won’t soon forget about those four months of misery. When the source of Lucy’s rash was finally identified and gluten was off the hook, she and her parents went out for pizza to celebrate.
“I was really happy that I could finally eat gluten,” she remembers of that celebratory slice.
And she’s got a message for companies that use MI in their products.
“I would tell them that that chemical should have never existed,” Lucy tells Consumerist. “And whoever made up that chemical should just go in jail.”
And while it was also hard for John to talk about Lucy’s awful experience, he says if it can bring the issue to light for other parents, he’s more than willing to remember every awful moment.
He explains, “Where I am now, I’m thrilled to share in the hopes that someone else won’t have to live through it.”
*We have changed these names in order to protect the privacy of Lucy, a minor.
Editor's Note: This article originally appeared on Consumerist.