Treating cancer can be a painful, drawn-out process, and there’s no guarantee of a cure. Unfortunately, there’s no magic pill that can get rid of cancer, or prevent it from occurring in the first place. That hasn’t stopped a number of companies from making unproven promises about products they claim will remedy everything from AIDS to diabetes.
[More]
food and drug administration

FDA Warns 14 Companies For Selling Unproven Cancer ‘Cures’

Senators Urge FDA To Ban Possible Carcinogen Found In Shampoo, Lotion
You might not be familiar with “1,4-Dioxane” but it’s a chemical component commonly used in everyday products, such as shampoos, lotions, and cosmetics. It may also cause cancer, which is why a pair of U.S. Senators are urging the Food & Drug Administration to begin the process of eliminating this chemical from consumer products. [More]

Trader Joe’s Applesauce Recalled Because Apples Don’t Contain Glass
There are number of things you can put in applesauce to give it a bit of a kick: cinnamon, brown sugar, but definitely not glass. Yet, Trader Joe’s is recalling its applesauce over concerns it might contain that dangerous, unwanted ingredient.
[More]

Legislation Would Give FDA Mandatory Authority To Recall Drugs
Last month, the Food and Drug Administration confirmed that it had found varying and elevated levels of a potentially deadly toxin in teething tablets sold under the Hyland’s brand. Despite the dangers posed by the tablets, the FDA couldn’t order a recall of the products — and the manufacturer refused to. But that could change in the future, as recently introduced legislation would give the agency the authority to order mandatory recalls of drugs and homeopathic products. [More]

A Supplement Company Sued Over Research It Didn’t Like… And Lost
Unlike FDA-approved medications, makers of dietary supplements are not required to demonstrate that their products are safe or effective. That shouldn’t stop independent researchers from doing their own tests to find out if a product works or is dangerous, but when one Harvard professor tried to do just that, supplement makers tried to shut him up.. [More]

FDA Recommends Limiting Lead In Lipstick & Other Cosmetic Products
Though no one really wants to hear they are putting lead into or on their bodies, the fact is that many cosmetics contain low levels of lead. While the amount of lead in your lipstick might be too low to do any harm, the Food and Drug Administration is still taking steps to further limit the amount of the chemical found in such products. [More]

Senator: Regulators Should Investigate E-Cigarette Explosions, Issue Recalls
Despite their popularity, e-cigarettes are a “ticking time bomb” that should be more closely investigated by federal regulators and recalled if necessary, according to New York Senator Chuck Schumer. [More]

Judge Gives Initial Approval To $26M Settlement For Claims That WEN Products Caused Hair Loss
In July, we heard that the Food and Drug Administration was investigating reports of hair loss, balding, itching, and rash associated with using celebrity stylist Chaz Dean’s hair care products, sold by a company called WEN. Now, a federal judge in Los Angeles has given preliminary approval to a $25 million settlement that resulted from a class-action lawsuit more than 200 customers filed against the company. [More]

Nutella’s Makers Want To Convince FDA That Chocolate Spread Isn’t Just For Dessert
When you think of sweet, hazelnut chocolate spread, are you imagining eating it for breakfast or for dessert? If you’re in the latter camp, you agree with the current stance of federal regulators. The makers of Nutella are now trying to make the case, however, that the spread should be considered more of a breakfast item. [More]

Good Earth Egg Products Recalled After Spate Of Salmonella Illnesses
Missouri-based Good Earth Egg Company has issued a recall for a variety of egg products that health officials have connected to an outbreak of salmonella in three states.
[More]

FDA Warns Dozens Of Retailers Caught Selling E-Cigs, Liquid Nicotine To Minors
The Food and Drug Administration recently finalized rules making it clear that e-cigarettes and liquid nicotine are, just like traditional cigarettes, not to be sold to people under the age of 18. Now the FDA is putting dozens of retailers on notice that they were caught allegedly selling these products to minors. [More]

Makers Of E-Cigarettes And Pricey Cigars Want To Avoid FDA Approval And Regulation
Until earlier this year, the Food and Drug Administration didn’t have authority to regulate some new or unusual smokeable products that have been growing in popularity, like premium cigars, hookah tobacco, vaping products, and e-cigarettes. However, the industries behind these products are fighting regulation with lobbyists, hoping to do away with the new rule. [More]
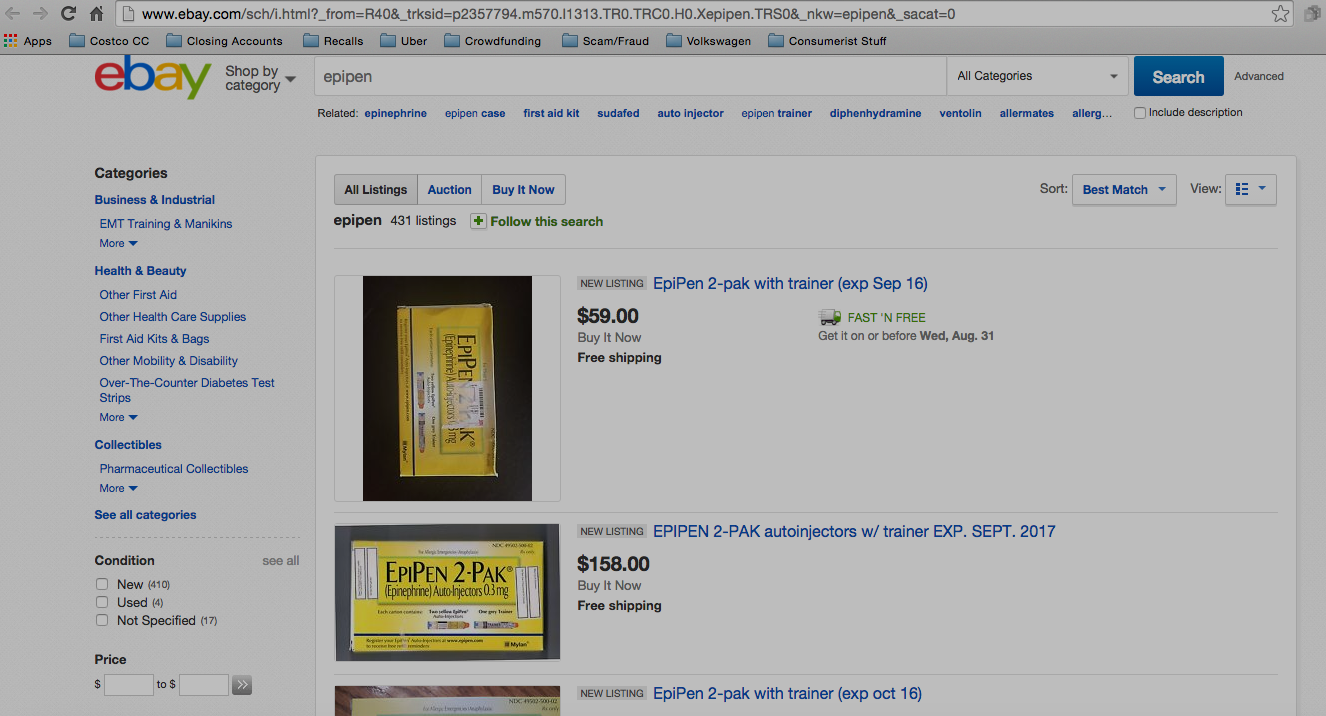
Regardless Of Price, Don’t Buy Your EpiPen On eBay
With the price of emergency allergy treatment EpiPen jumping nearly 600% in less than a decade, bringing the out-of-pocket cost for some patients to $600 for a two-pack, it’s perhaps not surprising that sketchy eBay sellers are claiming to offer the prescription medication at a discount, even though it’s against eBay policy, illegal, and just a really, really, really awful idea. [More]

The FDA Doesn’t Actually Have The Power To Recall Cosmetics That Harm People
Last month, the Food and Drug Administration announced that it would be investigating claims from consumers that the “cleansing conditioner” Wen, purportedly developed by celebrity hairstylist Chaz Dean, had caused scalp irritation and even made some users’ hair fall out. The FDA looked into the situation after receiving 127 complaints about the product, but didn’t know that the marketer, Proactiv maker Guthy-Renker, had received more than 21,000 complaints about the product that it wasn’t obligated to report to the FDA. [More]





