The Food and Drug Administration has asked Endo Pharmaceuticals, the manufacturer of Opana ER, an extended-release semi-synthetic opioid painkiller, to remove the drug from the market, after the agency concluded that the drug’s potential for abuse outweighed its therapeutic value. [More]
fda
FDA Requests Opioid Painkiller Be Removed From The Market, Citing Abuse Risks, HIV Outbreak

Cashews Sold At Aldi Recalled Because Glass Isn’t A Tasty Snack
If your afternoon snack today includes cashews you bought at Aldi, you might want to step away from the can: The packages of nuts have been recalled, as they could contain pieces of glass. [More]
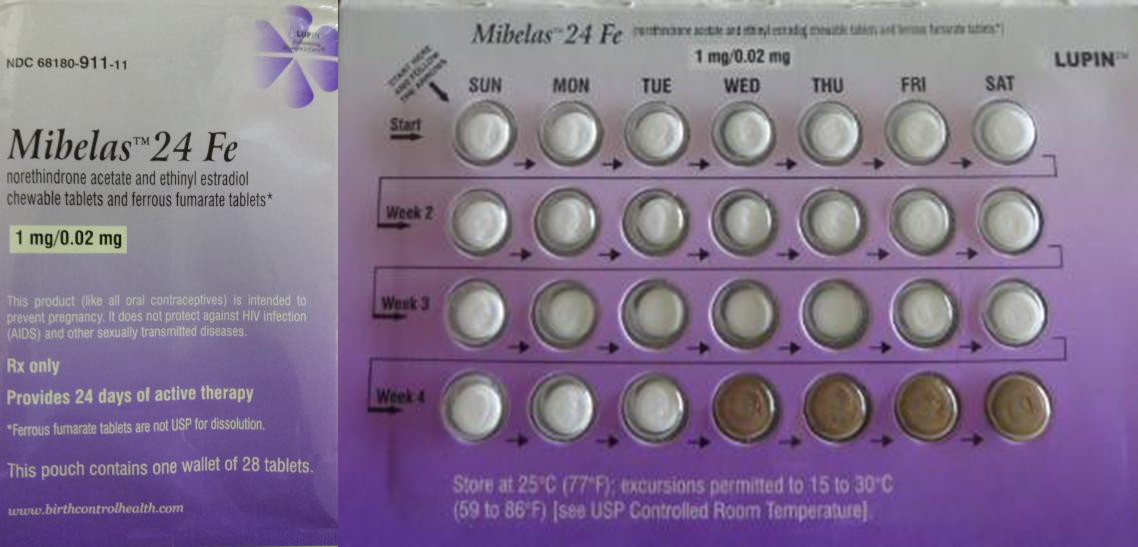
Out-Of-Order Birth Control Pills Not Great At Preventing Pregnancy, Prompt Recall
As anyone who’s ever used an oral contraceptive knows, it’s important to take the pills in the right order, because each dose is different: Some pills contain hormones and some are inert and don’t do anything. Mixing those up could mean the difference between remaining unpregnant and getting pregnant. To prevent the latter, the maker of one birth control product is recalling pills that are packaged incorrectly. [More]

Dumpster Diving For Beauty Products: Is It Legal And Safe?
Sure, it’s always nice to get a great deal on beauty products, and what’s a better deal than 100% off? “Dumpster Diving” — the art of sourcing still-usable items from the trash — is nothing new, but there are growing reports from beauty bloggers and YouTubers claiming to score free lipsticks, nail polishes, eye shadows, and other items by sorting through the items that Ulta, Sephora, and others throw out. Is this legal, and if so, is it safe? [More]
Diabetes Drug Invokana Must Warn Patients About Increased Risk Of Foot, Leg Amputation
A new class of diabetes medicines is heavily advertised on TV and shows great promise in getting patients’ blood sugar levels down, but a safety announcement from the Food and Drug Administration warns that one of the drugs, Johnson & Johnson’s Invokana, doubles those patients’ chance of needing parts of their legs or feet amputated. [More]
FDA, CDC Warn Families About Inaccurate Readings In Some Lead Tests
It’s easier than ever for parents (and parents-to-be) to get their lead levels tested, but federal officials are now warning American families that certain popular lead tests may provide inaccurate results, increasing the risk for lead poisoning in certain young children and adults. [More]

New Safety Risks Found In 1-In-3 Drugs After FDA Approval
No one wants patients to have to wait longer for access to potentially lifesaving new drugs, and the newly approved head of the Food and Drug Administration has made it clear that he intends to speed up current approval processes when possible. However, a new study says safety risks were found in around one-third of all new FDA-approved medications after they had been okayed for use by the agency. [More]

FDA Warns 14 Companies For Selling Unproven Cancer ‘Cures’
Treating cancer can be a painful, drawn-out process, and there’s no guarantee of a cure. Unfortunately, there’s no magic pill that can get rid of cancer, or prevent it from occurring in the first place. That hasn’t stopped a number of companies from making unproven promises about products they claim will remedy everything from AIDS to diabetes.
[More]

FDA Issues New Warning On Giving Kids Codeine Or Tramadol
The Food and Drug Administration warned that cough and pain medications containing codeine or tramadol should not be given to children after reports that the drugs caused life-threatening breathing problems. [More]

Sexual Enhancement Supplements With Ridiculous Names Contain Prescription Drugs For Sexual Dysfunction
A supplement company that must let their 12-year-old cousin name its products has recalled a slew of sexual enhancement supplements — for men and women — after tests showed that these items contained actual FDA-approved prescription drugs used to treat sexual dysfunction. [More]

Hyland’s Officially Recalls All Homeopathic Baby Teething Tablets Over High Levels Of Belladonna
Even though the Food and Drug Administration warned the public in 2016 about elevated levels of belladonna, a potentially dangerous toxin, in homeopathic teething tablets produced by Hyland’s — and then confirmed this risk in January — the products remained on store shelves and in family’s medicine cabinets. Now, under pressure from the FDA, Hyland’s has officially issued a recall for all of its homeopathic teething products. [More]

Fresh Express Recalling Salad Mix Sold At Walmart After 2 People Find Dead Bat In Their Food
If you were about to eat something, you may want to put it down for just a second. Now pick it up and take a closer look at it: Any dead animals that shouldn’t be there? No? You’re luckier than two people in Florida who found a dead bat in the packaged salad they were in the process of consuming. [More]

When Does The Extra Space In Your Potato Chip Bag Go From Annoying To Deceptive?
If you have two bags of potato chips with the same size packaging, containing the same weight of potato-based snacks, does it matter if one of those bags also contains more empty space? It does to two customers who have accused Wise of misleading shoppers by selling bags of snacks with lots of air. [More]
Some EpiPen, EpiPen Jr. Devices Now Being Recalled In U.S. Because They May Not Work When Needed
Mylan, the makers of the EpiPen emergency allergy treatment are expanding a previously announced overseas recall to now include EpiPen and EpiPen Jr. devices distributed in the U.S. over concerns they may not function properly when needed. [More]





